Chewing Gum and Helium Viral Video
http://www.hoax-slayer.com/gum-helium-experiment.shtml
Much like another ad with young fools blowing up denim jeans with helium.
Chewing Gum and Helium Viral Video
http://www.hoax-slayer.com/gum-helium-experiment.shtml
Much like another ad with young fools blowing up denim jeans with helium.
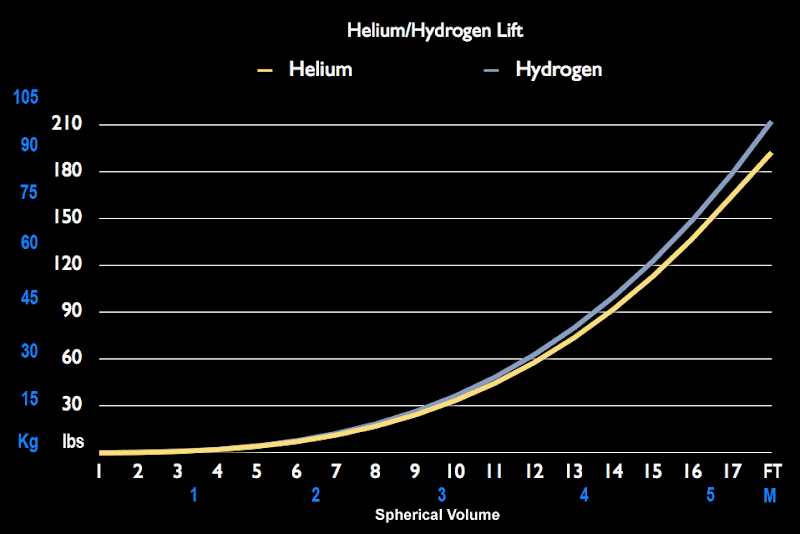
What kind of lift would this have compared to hydrogen?
In the article, it claims you would need about 4,000 helium balloons to lift a 110 lb. person. Now, that being said, would it be possible to condense that amount of helium into a smaller package, say a 8ft sphere?
That wouldn't do you any good. The reason helium has lift is because it's less dense than air at the same pressure. Compressing it would increase its density and negate the buoyancy.
What kind of lift would this have compared to hydrogen?
It's not much of a difference. Hydrogen only provides about 8% more lift than helium.
What I always find amusing about these kinds of videos, especially when I don't think about them myself, are the obvious flaws that people just overlook. For instance, in this video a person can blow a bubble with gum, and it supposedly would then be strong enough to carry that person without stretching or tearing. They did a good job on the video, and the viral advertising is a good idea, although I hate how so many things inevitably are used for ads.
Helium or even hydrogen would be needed in huge quantities to provide any lift where the medium has a density close to air at sea level and with Earthlike gravity, but those are not the only options. You can go two ways with it. One is to consider being on a planet or moon with much lower gravity than here. I worked out that Enceladus, a satellite of Saturn, might be an appropriate environment given an environment with sea level type air pressure and hydrogen was used.
The other way to go would be to have a denser medium. Being underwater is an obvious example but rather less than fun because human bodies float in that anyway. However, particularly dense gases or high pressure outside the body, such as xenon or sulphur hexafluoride in the air, would help swing it. Provided at least 200 millibars of that pressure is provided by oxygen the atmosphere would still be breathable. A combination of low gravity and dense atmosphere would make it less extreme.
So i would say it can be done in special circumstances. The closest you could get to them would probably be on Titan or possibly Venus (though Venus is ridiculously hot!). A malfunction of a hydrogen or helium cylinder on the surface of Venus is a possible scenario, provided the person concerned was wearing a REALLY effective environmental suit which was also very stretchy.
viral advertising is a good idea, although I hate how so many things inevitably are used for ads.
How about an inflation ad? Suppose some viral marketer decided to use inflation in some way to push a product -- even something as overt as placing the product logo on the inflated person like the Goodyear blimp. Imagine the production value...
In the article, it claims you would need about 4,000 helium balloons to lift a 110 lb. person. Now, that being said, would it be possible to condense that amount of helium into a smaller package, say a 8ft sphere?
Needs to be a bigger volume. :)
I went through the math a while a go - Helium provides something like 0.03lbs of lift per cubic foot of volume @ STP. To lift myself, and the weight of the envelope, would require a balloon around 30' in diameter (which, if the 0.03 number is correct, would lift 424#).
If one balloon is approximately 1 cu. ft., then to lift one pound would require 34 of them. For 110#, that would be 3,740 balloons. Feeding 3740 into 4/3 * PI * R^3, and solving for R comes up with a radius of 9.6 feet, or a balloon 19 feet in diameter.
I fully admit my math may be faulty, and those numbers might be completely off base, so YMMV.
Kryslin
Interesting find, BlankSlate!
I can imagine now how big the inflation subject would have to be to be able to fly. And thinking about it makes me feel all warm and fuzzy inside.
Helium or even hydrogen would be needed in huge quantities to provide any lift where the medium has a density close to air at sea level and with Earthlike gravity, but those are not the only options. You can go two ways with it. One is to consider being on a planet or moon with much lower gravity than here. I worked out that Enceladus, a satellite of Saturn, might be an appropriate environment given an environment with sea level type air pressure and hydrogen was used.
If the gravity is lower, the atmospheric pressure would also be lower at sea level.
Lift is how much less a given volume of one gas weighs than what it displaces.
Helium or even hydrogen would be needed in huge quantities to provide any lift where the medium has a density close to air at sea level and with Earthlike gravity, but those are not the only options. You can go two ways with it. One is to consider being on a planet or moon with much lower gravity than here. I worked out that Enceladus, a satellite of Saturn, might be an appropriate environment given an environment with sea level type air pressure and hydrogen was used.
Unfortunately air pressure will be proportionally (to g) less there. Then same volume of helium will be needed.
By the way:
Helium is 4g per mole, while air is 29g per mole. Difference is 25g per mole. One mole of gas takes 22.4 liters of volume in normal atmospheric pressure.
If we are going to lift a 50kg girl (rather skinny), it will take 2000 moles or 44800 liters, or 44.8 cubic meters of helium. Thats sphere with radius 2.2 meters (or aprox 7 feet).
I hate how so many things inevitably are used for ads.
They have the budget to make videos like this.
I imagined an enclosed habitat containing a breathable atmosphere, which is why i didn't consider the density of the real atmosphere in those places except for Venus and Titan.
A human filled with gas won't have that gas at the same molarity as the surrounding atmosphere. It will be under pressure. If the person in question weighed fifty kilos and also had extremely baggy skin or internal organs initially, they could be filled with a lighter than air gas at comparable pressure to the surroundings. The same would, interestingly, apply to a large, baggy garment, though it would have to be huge for that to make a difference.
However, we are elastic and our organs, body walls or skins therefore applies pressure to the gas inside us. This means that any inflating gas will be under pressure pretty soon, before it causes any distension. Incidentally, that also means it would cut off the blood supply after a certain point, somewhat above atmospheric pressure and further above it than physiologically due to adrenalin. When Denise Nickerson wore the inflation suit in 'Willy Wonka', she had to be shifted about because it was cutting off her circulation.
When Denise Nickerson wore the inflation suit in 'Willy Wonka', she had to be shifted about because it was cutting off her circulation.
Nickerson wore two different blueberry costumes. The first costume in the scene was inflatable. The second costume, when Violet appeared fully inflated, was actually a giant styrofoam ball. The two hemispheres of styrofoam were hollowed out to make space for Nickerson. It was very uncomfortable, as Nickerson had her arms folded up to put her hands in the right place.
Shooting the blueberry scene was apparently an all-day affair, so Nickerson got cramps from holding the same pose for so long. The costume also had weights added to the bottom to keep her from rolling over, which added to the discomfort.
Thanks for that. I knew about the two separate costumes but not that much detail. It's very interesting. So was it the polystyrene one which was weighted or the inflatable suit?
I've thought about this rather a lot!
I still think inflation would impair blood flow. Suppose that with the adrenalin the systolic blood pressure is 250 mm Hg. That's equivalent to 1.3 bars. That means that once pressure within the organs or body cavities got beyond 1.3 bars, which isn't very high, the heart wouldn't be able to push the blood through the arteries to the rest of the body. So you would first of all get a throbbing sensation, then pain from poor blood supply, then tingling like pins and needles when an arm has been slept on, then numbness as the blood supply gets completely cut off. That would also mean the musculature would relax, because the ability to release energy to maintain muscle tone would be lost. That relaxation would lower the pressure slightly and maybe allow blood to flow again, so i think there'd then be a relaxation allowing more inflation, then a stage of tension, then more relaxation and so on. Eventually the pressure would just be too high all the time.
The inflatable costume was pleated and held together by the belt. The pressure was probably not even as constricting as a neoprene wetsuit -- which a friend once described as feeling like "god checking your blood pressure." Although Paris Themmen as Mike Teevee gave Nickerson a good poke in her inflating belly, offsetting her balance. The shot cuts right at that point, and I wonder if Nickerson fell over?
BLOOMP! "Cut! Cut! We're not ready for the rolling yet!"
Oh, good question, i never noticed that before. Isn't it amazing how you can watch something hundreds of times without noticing a detail like that?
It's pleated but looks to me like it's got something quite thick underneath it. It's not like it's just a binliner and i think it would've been quite elastic and resilient just by the look of it.
Completely agree with the blood pressure thing, which brings up another point: would the pressure force blood into the extremities, for instance the genitals?
In the article, it claims you would need about 4,000 helium balloons to lift a 110 lb. person. Now, that being said, would it be possible to condense that amount of helium into a smaller package, say a 8ft sphere?
The question has been fielded, but I wanna' take a stab at it anyways to give some more intuition as to how/why.
Answer: No.
Helium gets its lift by displacing air. You float in water because you weigh less than the water you displace. If you're heavier than the water you displace (ie. made of rock), then you sink.
Air behaves the same way. If you force helium into a small container, it keeps the same weight. It becomes denser. Subsequently, it displaces less air. Same weight, less air displaced (and subsequently lower weight of air displaced), means less likely to float.
If helium simply floated because it was helium, the cylinders full of compressed helium would be skyward in no time flat. They don't float because there's so much helium smooshed into them that it's denser than air.
I completely agree. All ordinary matter has weight. Hydrogen and helium are simply the lightest forms of matter. Air is heavier than them. This would only work if physics were Aristotelian, with certain substances exhibiting levity and others having gravity. There are ways round it but there's no way it could happen inside a normal human body in normal conditions.
Anti-Gravity paint would help. Just ask Danny Dunn.
In the article, it claims you would need about 4,000 helium balloons to lift a 110 lb. person. Now, that being said, would it be possible to condense that amount of helium into a smaller package, say a 8ft sphere?